Thorium
| Thorium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General Info | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Symbol | Atomic symbol::Th | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Number | Atomic number::90 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Weight | Atomic weight::232.0381 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical series | Actinide | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | ? 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | none, 7, f-block | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Rn] 6d2 , 7s2 , | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 18, 10, 2 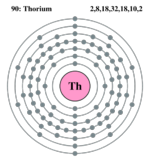
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | CAS number::7440-29-1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density | Density::11.78 g/ml | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | Melting point::1750° C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | Boiling point::~4790° C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes of Thorium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| All properties are for STP unless otherwise stated. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Thorium is a chemical element with the chemical symbol of Th, which is classified as a transition metal and an actinide. It is a rare radioactive metal, that was named after Thor, the mythological Scandinavian god of wartakes. It is a great source of nuclear power and and more abundant in the earth's crust than uranium. It was first discovered in 1829 by Jöns Berzelius in Sweden, when he was handed a mineral that had thorium inside of it. It was later learned about thorium decay contributes to the internal heat of the earth. Thorium can be found in thorite and thorianite located in New England, and many other sites.[1] Some of the elements in the mineral that Berzelius received were iron, manganese, lead, tin, and uranium. There was one more substance in it that he could not identify, which turned out to be thorium. He first called the black mineral that he found thorite, then he indicated that 57.91% of thorite was an oxide, so he named it thorium. But it wasn't until 1898 when Gerhard Schmidt recorded that it was radioactive. It is also a very promising element because it can be found pretty easily, and can produce as much energy as 200 tons of mined uranium. [2]
Properties
The pure form of thorium is a silvery-white metal which sustains its luster for several months, and is air stable. But when thorium is contaminated with oxide, it turns gray, then finally black. The degree of oxide greatly influences the physical properties, and how it is shaped. The pure form of thorium retains a soft, and very ductile form that can be cold-rolled, swaged, and drawn. Thorium is dimorphic, changing at 1400 C from a cubic to a different structure that is body centered. The thorium oxide has the melting boiling point, which is 3300C, and that is higher than all other oxides. Thorium does not dissolve easily in most common acids, except hydrochloric. The powdered thorium metal is pyrophoric and should be handled with delicate care. Thorium then burns brightly when it is heated, and turns into ignite. [3] When it is heated, a very bright white light will appear. The powdered thorium really needs to be handled with care because it is pyrophoric and could be very dangerous. The specific gravity of thorium is 11.72 and has a valence of +4. The physical properties of this metal depend highly on the oxide that is present. It will then disintegrate to produce radon gas, an alpha emitter and radiation hazard, so the areas where it is stored requires specific and protective ventilation. [4]
Occurrences
Thorium comes very abundantly in the earth's crust, but is mostly found in small amounts. It is three times more abundant than uranium, and there is almost as much found as lead. Granite can obtain up to 80 ppm of thorium but it can also be found in most rocks and soil. Thorium does not really circulate through the environment, but it does occur naturally in thorite, uranothorite, thorianite, and is a huge component of monazite. The production of thorium is rising, which means that the increase of thorium is rising to. Simply because accidental releases of thorium happen all the time on the processing plants. The world production of thorium, 30,000 tons per year and the reserves, that are known exceeds 3 million tons. [5] Thorium can be more useful than Uranium in some very great ways. Since Thorium occurs so much, and it is cheaper than Uranium, it could be used to make electricity and replace more of Uranium in the world. Monazite is the most common ore and is very abundant, unlike the others. Monazite is a form of beach sand that you walk on occasionally. It is found predominantly in Florida. The sand can contain up to ten percent Thorium. [6]
Uses
Thorium can be a source of nuclear energy and be very helpful to our world. Portable gas lights have mantles that consist of Thorium oxide (ThO2). It has about one percent cerium oxide, and a couple other ingredients. When it is put in a gas flame that is heated, it will glow with a dazzling light. Another use of this Thorium oxide is used to coat tungsten wires that function in electronic equipment. It does this because it has a low work-function and a high electron emission. Another use is in electric lamps, the oxide is used to control the grain size of the tungsten that is in it. Thorium oxide has a high refractive index and low dispersion, which makes it appear in glasses and other objects. This is why scientists use it in high quality cameras and scientific instruments. Another use of the oxide would come in the form of a catalyst, for the ammonia to nitric acid, petroleum cracking, and sulphuric acid production. Scientists use it because it is used for high temperature laboratory crucibles. [1]
Some use of Thorium is used as an alloying agent to improve the strength of magnesium at high temperature. Also when Thorium gets bombarded with neutrons, the thorium 232, becomes thorium 233, that eventually decays into uranium 233 through series of beta decays. The Uranium 233 then becomes fissible and can be used as a nuclear fuel. But the primary use of thorium known today is in lantern mantles. It is primarily known as Welsbach mantle and grows to a bright white light when heated to high temperature. [7]
Environmental and Health Effects
Since thorium reacts slowly with water, oxygen, and other compounds, it forms a variety of tungsten compounds. Some effects on plants or animals would be that too much thorium released could effect and contaminate the plants and animals. Also, the product and form size of no unusual environments really could effect the animals, but it is not expected. The contamination will not be bad unless it is in a large release, which affects the water and the aquatic animals. It has to be handled carefully when disposing of it, and be legal to get rid of it in some places.
The health effects could be a lot worse though, due to the amount of thorium around us.It can be found in small amounts in the air, our food, and water. The inhalation of air though is not that bad in very small amounts and can be ignored. When uncontrollable amounts are found they can be blown around in the wind and be in someones food, that lives or was by a uncontrolled site. If you are on the work site of thorium and inhale it, you could get lung disease, lung cancer, and pancreas cancer. The effects will take place years after the exposure happens. It also has the ability to change genetic material, and develop liver disease if you are injected with special thorium x-rays. Since it is radioactive to, it can be stored in bones and cause bone cancer. Then some people die of too much exposure of breathing thorium, due to metal poisoning.[8]
References
- ↑ 1.0 1.1 Winter Mark. Thorium uses Webelements. Web. Accessed 11/3/11
- ↑ Thorium Element Facts Chemicool. Web. 6 June 2011. Unknown Author
- ↑ Unknown author, Thorium uses Periodic table of elements. Web. Accessed 11/17/11.
- ↑ Helmenstine Anne Marie, Ph. D., [1] Thorium Facts. Web. Accessed 10/21/11.
- ↑ lenntech B.V. Thorium-Th Water Treatment Solution Lenntech. Web. Accessed 10/27/11
- ↑ Unknown Author THORIUM Chemistry Explained. Web. Accessed 11/2/11
- ↑ Gagnon, Steve. The Element Throium education.jlab. Web. Accessed 11/3/11
- ↑ Unknown author Thorium Th Lenntech. Web. Accessed 11/17/11
| ||||||||||||||
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||


