Sulfate
| Sulfate | |
|---|---|
| |
| General | |
| Systematic name | Sulfuric acid |
| Other names | hydrogen sulfide |
| Molecular formula | SO42− |
| SMILES | O=S |
| Molar mass | Molar mass::96.06 g/mol |
| Appearance | white precipitate |
| CAS number | CAS number::Not Available |
| Properties | |
| Solubility in water | 250mg/l |
| Melting point | Melting point::3°C |
| Boiling point | Boiling point::290°C |
| Structure | |
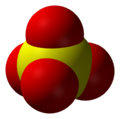
| Molecular shape | Tetrahedral arrangement |
| Hazards | |
| MSDS | Material safety data sheet |
| Main hazards | Ingestion |
| Related compounds | |
| Related sulfur oxoanions | Peroxomonosulfate Sulfite Pyrosulfate Dithionate Metabisulfite Dithionite Thiosulfate Tetrathionate |
| Related compounds | calcium sulfate strontium sulfate lead (II) sulfate barium sulfate |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Disclaimer and references | |
Sulfate is an inorganic polyatomic anion composed of sulfur and oxygen. It has an empirical formula SO42− and forms salts with many metals. It occurs naturally in drinking water and health concerns regarding its ingestion are now a concern of the U.S. Environmental Protection Agency.[1]
Properties
Sulfate is a salt of sulfuric acid. Most are highly soluble in water, for example, calcium sulfate, strontium sulfate, Lead(II)sulfate, Barium sulfate, and Radium sulfate. Sulfate is work on conjugate base so it can be together with other elements or compounds. However, these compounds doesn’t perfect highly soluble in water, some compounds are poor soluble in water. For example- calcium sulfates storntium sulfate, lead (II) sulfate, and barium sulfate. The other side, Radium sulfate is best example which is soluble in water. Sulfate is on sulfur oxoanions list.
Sulfur oxoanions list = peroxomonosulfate, sulfate, sulfite, pyrosulfate, dithionate, metabisulfite, dithionite, thiosulfate, and tetrathionate. [2]
Occurrences

Mostly, sulfate was found in natural water (groundwater), because nature water contains high percent of sulfate. In water, we can get sulfate through three ways, which are reverse osmosis, distillation, and ion exchange. The reverse osmosis way is use treatment system, and then ejects most elements which are sulfate and others. The distillation way also use water treatment system, then boiled water, and it will removed without sulfate and others, so sulfate will remain on boiling pot. The last way is Ion exchange way, it is pulling out the quantities of sulfate from the water. But it doesn’t use in house, it only work for chemical working. [3] In other compounds, we can also find the sulfate. The Magnesium sulfate (MgSO4), Calcium sulfate (CaSO4), Barium sulfate(BaSO4) and Strontium sulfate (SrSO4), these four compounds contain sulfate. So we can separate the sulfate from other elements. For example, the calcium sulfate is come from natural state, so we can separate sulfate and calcium. Also barium sulfate could be separated with sulfate and barium.[4]
Uses
Sulfates have many uses in our lives such as the lead-acid battery wherein sulfate from sulfuric acid forms an electrolyte between lead metal (Pb) and Lead (IV) dioxide (PbO2). In micro organisms, sulfate is used as an electron acceptor. Well known, Epsom salts is sulfate combined with magnesium to form magnesium sulfate, which is mostly used for saline laxative. [5] in addition, the morphine sulfate is used for medicine for patients. In hospital, morphine sulfate used to control the pain. It can control on short term pain and long term pain. Also morphine can anesthesia ion surgeries and control hard breath. It can help to dying person, because it can make person’s body to be relaxed. However, sometimes, it used to drug, some drug addict come to hospital to take morphine without pain. [6] The ammonium sulfate is used to fertilizer to alkaline soils. It has lower PH lever, so if it was put on alkaline soils, it can make ph balance to be natural levels. Also it is support on herbicide and insecticide on agricultural parts. Aluminium sulfate is used for clean the water. In water, it works to make congelation of impurities, and clean on the bottom parts of water tank.
Hazard and First Aid
The natural water contains high concentrations of sulfates, so we need be careful about ingestion and inhalation. In 1993, World Health Organization made limit about of sulfate, it was 500mg per 1L, but after 1998, it changed 250mg per 1L. Sulfate doesn’t dangerous, but if Kids take many sulfate, it can cause death and diarrhea. If you ingest over 500mg of sulfate, you need drink water to dilution of sulfate and take medicine. If sulfate contact skin and shows irritation, need clean you skin with soap with water and take medicine for that. If sulfate contact to eyes, it can change to red, when the eyes was changed to red, you need clean up your eyes with flow water over 15min. if it doesn’t change or has more problem, you need go to department of ophthalmology. [7]
References
- Sulfate Wikipedia.org, Wikimedia Foundation, Inc
- Sulfates lenntech, water treatment and purification unknown author.
- The Sulfates Class Mineral Gallery unknown author.
- Sulfuric Acid MSDS, Environmental Health & Safety, .
- History of sulfate SAO/NASA
- Magnesium sulfate wikipedia.org, wikimedia foundation, Inc
- Morphine sulfate university of maryland medical center unknown author.
| ||||||||||||||

