Rhodium
| Rhodium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General Info | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Symbol | Atomic symbol::Rh | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Number | Atomic number::45 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Weight | Atomic weight::102.9055 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical series | transition metals | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | red powder 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 9, 5, d | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Kr]5s14d8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2,8,18,16,1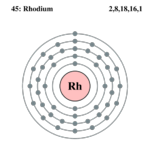
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | CAS number::7440-16-6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density | Density::12.45 g/ml | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | Melting point::1964 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | Boiling point::3695 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes of Rhodium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| All properties are for STP unless otherwise stated. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Rhodium is a chemical element found on the Periodic Table of Elements in group VIIIb, and on the 5th period. This element has an atomic number of 46, and is a part of the transitional metals. The symbol used for Rhodium is known as Rh. The position of rhodium on the period table, states that its valence electrons are filled up to its fith valence shell, which are 2, 8, 18, 16, 1. This electron configuration fills up to its ninth, 4d sublevel.[Chemistry for Christian Schools]
Rhodium was first discovered by an English Chemist, Wiliam Hyde Wollaston, in 1804. He found rhodium within palladium, by extracting platinum and palladium resulting in red colored salts by mixing hydrogen gas with it.[1] For its shiny and reflective hue it is suitable for manufacturing jewelry, cameras, wires, electrical contacts, electrical ducts, and other items. Rhodium is a valuable metal due to its durability, chemical, heat and electrical contact ability.[2]
Properties
Rhodium, which has a shiny silvery white hue, is part of the platinum group of metals (also known as the 'PGM' group). Compared to platinum, its melting point is higher and it has a lower density. Rhodium is rather hard and durable, it is not very malleable.[3] When heated, it will react and become an oxide which is red in color. Rhodium is flammable, and dust explosions are possible if it is powdered in oxygen.[4]
In order to make the metal rhodium, there must be a separation and removal of other metals from the ore, such as silver, gold, palladium, and platinum. It is then disjointed from the other metals. It is melted with sodium bisulphate, extracted with water, and becomes rhodium sulphate. It is precipitated as the hydroxide by adding sodium hydroxide , where its redissolved in hydrochloric acid to give H3RhC16. And so, it is precipitated into rhodium complex. This rhodium precipitate evaporated and reacted with hydrogen gas results in pure elemental rhodium.[5]
Occurrences
Found in diverse countries and locations, Rhodium is found abroad. Rhodium is found in North America, Canada and is also found in Africa, a completely different continent. Rhodium can be found in association with other metals such as platinum and palladium. Due to the difficulty in seperating Rhodium from these metals it is less economical than could be. [6] However, it is even found in diverse areas of the world such as, Brazil, Colombia, Russia, South Africa, and Sri Lanka.[7] Rhodium is the hardest to find of metals, because it is not a pure metal alone, but has to be seperated from others. South Africa has a significant amount of Rhodium, it has 60 % of the world's source of this element. About seven and eight tons of Rhodium is produced each year, all over the world.[8]
Uses
Rhodiums characteristics allow it to be used in electrical devices, high temperature alloys, specialized laboratory devices, thermocouple elements and in aircraft spark plug electrodes. These are all very useful instruments. Because of its hard and durable shiny aspect it is easily and commonly used in camera pieces, and silverware. Aside from Platinum and Gold, Rhodium is a most expensive and accessory of wealth. A hard optical flat mirror can be made out of Rhodium. It is used in everyday life, and has found much importance because of its lasting hard shiny characteristics.[9] Rhodium is used also, to make decorations and even observable objects in addition to being a catalyst.[10] Rhodium is a secondary production of Platinum mining and Nickel mining. Due to it's shininess it is used to make jewelry sparkle.[11] Around the 1930's Rhodium was extensively used in silverware, and in sterling flatware, it was so shiny that it didn't need to be shined frequently. During World War II, it was used over base metals. Great advancement in technology allows even thinner layers that are bonded on to the surfaces of other metals because of its good quality.[12]
Investment
Due to rhodium's hard and shiny quality, it is in great demand by automobile industries,. It is also used as a symbol of wealth. Since 2003, the use of rhodium has increased. It is expected that Rhodium may exceed $3,224.51 per ounce. Since it can be used in catalytic converters ofr diesel cars, its price has increased. Europe has a great demand for this shiny metal because it has no substitute.[13]
References
- Rhodium: the essentials Unknown Author, University of Sheffield, Unknown Date.
- Rhodium Unknown Author, University of California, 12/15/2003.
- Rhodium - Rh Unknown Author, Lenntech Water treatment & purification Holding B.V , Unknown Date.
- Rhodium – Discovery, Occurrence, Production, Properties and Applications of Rhodium aZoM.com, AZoNetwork, 7/7/2006.
- Rhodium Uses Unknown author, Precious Metal Investment, 09/07/09.
- Uses of Rhodium Unknown Author, Unknown Publisher, Unknown Date.
- FAQ Knowledge Base Unknown Author, Ruby Lane Advantage, Unknown Date.
- Platinum-Group Metals Statistics and Information USDA, Public domain, Unknown Date.
- Rhodium Investment: The Rarest of Precious Metals Brad Zimmerman, Nu Wire Investor, 04/10/2008.
- The Element Rhodium Unknown Author, Jefferson Lab, Unknown Date.
- Rhodium- Brian R. James, Advameg, inc., Unknown Date.
External links
- H. CROSS COMPANY Unknown Author, H. Cross Company, Unknown Date.
| ||||||||||||||



