Hafnium
| Hafnium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General Info | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Symbol | Atomic symbol::Hf | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Number | Atomic number::72 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Weight | Atomic weight::178.5 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical series | Transition metals | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | lustrous, silvery, ductile metal 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 4, 6, d | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Xe] 4f14, 5d2, 6s2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 10, 2 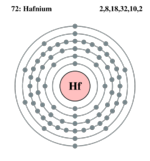
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | CAS number::7440-58-6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density | Density::13.07 g.cm-3 at 20°C g/ml | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | Melting point::2200°C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | Boiling point::5200°C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes of Hafnium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| All properties are for STP unless otherwise stated. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Hafnium is a chemical element with 72 of atomic number and symbol of Hf. This element has shiny, lustrous, silver appearances and also chemically ductility. It has hexagonal crystal structure and it is distinguished as a transition metal. In 1869, Dmitri Mendeleev predicted the existence of Hafnium, but finally Dirk Coster and George Charles de Hevesy discovered it in 1923 in Copenhagen, Denmark. Derived from Latin name of Copenhagen, the element receives the name, hafnium.
The characteristics of hafnium are absorption of neutrons and capture cross-section of large neutrons. Through these characters, hafnium is widely and used in various fields. It is commonly used in electrode, nuclear power plants, and nuclear reactors.
However, it is hard to get pure hafnium from zirconium minerals. Hafnium consists 0.00058% of the Earth's upper crust, but it does not separate easily from zirconium since they share similarities chemically. The only difference is their densities.
Properties
Physical Properties
Hafnium is a lustrous and silver metal with great ductility. Its atomic number is 72 and atomic mass for 178.49 g.mol-1.[1] This silvery metal is intimately related to zirconium which makes hard to separate hafnium from it. Impurities of zirconium influences the physical properties of hafnium much. Hafnium and zirconium have same number of valence electrons which is 2, and belong to same group, 4. The only difference between two similar metals is that zirconium's density is a half of that of hafnium. Also hafnium has a closely adhered hexagonal crystal structure, which is impenetrable and tough.[2]
Chemical Properties
Due to its tightly packed structure, hafnium resist corrosion better that other elements. Hafnium does not readily react with acids neither highly concentrated alkalis but by only hydrofluoric acid. However, it can be burnt in air or oxidized with halogens. When it directly reacts with halogens, it forms tetrahalides.[3] Moreover, it reacts with other atoms such as carbon, boron, nitrogen, oxygen, silicon and sulfur in high temperatures.[4]It also alloys with as iron, niobium, tantalum and titanium. It shows good absorption cross-section for thermal neutrons which is 600 times than that of zirconium. It has ability to absorb neutrons. [2] As zirconium has similarities with hafnium, they are closely related to each other chemically either. Only three characteristics of those metals are different, which includes melting points, boiling points, and solubilities. However, hafnium is not very reactive like zirconium is. Moreover usually in the air and in water or cold acids, hafnium and oxygen do not react with each other.
Discovery of Hafnium
In 1869, the periodic table made by Mendeleev predicted the existence of titanium and zirconium. However, Mendeleev did not predict hafnium first, so he placed lanthanum in spot of atomic number of 72 in 1871. [5] About 100 years later, Henry Moseley predicted the existence of hafnium in 1914 and finally physicist Dirk Coster(1889-1950) and chemistGeorge Charles de Hevesy(1889-1966) discovered the element in 1923 in Copenhagen, Denmark. Later, it was found in a piece of Norwegian zircon with other mineral zirconium. The discovery by the physicist and chemist is worked through X-ray diffraction analysis. After discovery, the two scientists named hafnium followed by the ancient name of Copenhagen. [6] X-ray diffraction analysis helps scientists figure out another ways to identify new elements. At a metal plate, electrons are burned and then the metal plate produce X-rays. However, the kind of metal plate used decide the kind of X-ray which it produces. Every metals has its own pattern of X-ray and that fact makes it able to distinguish the kind of metal and X-ray. Before the X-ray diffraction analysis is developed, scientists have found hafnium but consider it as zirconium. As the new X-ray method is developed, the discovery of hafnium finally happens.
Jantzen and de Hevesy repeated recrystallization of double ammonium or potassium fluorides to separate hafnium from zirconium. Specially, metallic hafnium is made by exposing hafnium tetra-iodide vapor to a tungsten filament with heat. The method to separate hafnium from zirconium is also commonly used in nowadays. [7]
Hafnium's name in other languages
•Latin: Hafnium
•Czech: Hafnium
•Croatian: Hafnij
•French: Hafnium
•German: Hafnium - s
•Italian: Afnio
•Norwegian: Hafnium
•Portuguese: Háfnio
•Spanish: Hafnio
•Swedish: Hafnium [8]
Occurrences
Although natural zirconium compound consists of hafnium, hafnium does not exist freely in nature. Hafnium is also found in other minerals like alvite [(Hf, Th, Zr)SiO4 H2O], thortveitite [(Sc,Y)2Si2O7] and zircon (ZrSiO4) about 1 to 5%. Estimately hafnium is abundant about 5 parts per million which is much as bromine, tin, and uranium. However, Hafnium and zirconium share chemical similarities and that makes it hard to separate those two atoms. As a by-product of zirconium refinement, hafnium is produced through decline of hafnium(IV) chloride with magnesium or sodium in the Kroll process, a pyrometallurgical industrial process used to produce metallic titanium.
Trachyte tuffs is the potential source of hafnium and the major sources of it are heavy mineral sands ore deposits from especially in Malawi and Brazil.
Also, hafnium composes about 0.00058% of the Earth's upper crust.
[7]
[9]
Purification
The main source of hafnium is getting hafnium-free zirconium. Zirconium has good chemical stability at high temperatures and low neutron capture cross-section. However, since hafnium contains neutron-absorbing properties, impurities of hafnium with zirconium does not help the efficiency. The separation of two atoms is necessary and also helpful for use in nuclear power.
The first methods used to separate hafnium from zirconium are fractionated distillation of the chlorides and fractionated crystallization of ammonium fluoride salts. However, they are found to be not suitable. In the 1940s, zirconium became a factor for nuclear reactor programs, which promoted another method for separation. The method developed later is liquid-liquid extraction processes.
In the Kroll process, hafnium chloride with double magnesium at 1100 °C is converted to magnesium chloride and pure hafnium.
HfCl4 + 2 Mg (1100 °C) → 2 MgCl2 + Hf
Moreover, Arkel and de Boer developed further purification. Hafnium with double iodine at temperature of 500 °C produces hafnium(IV) iodide. The reverse reaction occurs at a tungsten filament of 1700 °C, and hafnium is freely separated from iodine.
Hf + 2 I2 (500 °C) → HfI4 HfI4 (1700 °C) → Hf + 2 I2
Chemical Compounds
Hafnium diboride (HfB2)
Hafnium diboride has a hexagonal crystal structure with 10.5g/cm3 of density and 200.11 g/mol of molar mass. Its melting point is about 3250°C. It is a grey and metallic ceramic unlike other polymer and composite materials. It has relatively high electrical and thermal conductivities and it allow to use this compound in hepervelocity vehicles like ICBM heat shields. To strengthen its powder, hafnium diboride sometimes combines with silicon carbide, nickel, boron, carbon, or silicon. Through hot pressing, the powders are forced to gather tightly with heat and pressure, and form a solid. The another possible usage of the compound is for nuclear reactor control rods. Also,it is used as a microchip diffusion barrier, which can be 7 nm think or less than that. [11]
Hafnium(IV) carbide (HfC)
Hafnium(IV) carbide has a cubic crystal structure with 12.2g/cm3 of density and 190.50g/mol of molar mass. Its melting point is over 3890°C. This black odorless powder is one of the most refractory binary compounds, but it has relatively low oxidation resistance. It is insoluble in water. [12]
Hafnium(IV) oxide (HfO2)
Hafnium(IV) oxide is white powder with 210.49g/mol of molar mass and 9.68g/cm3 of density. It starts to melt at temperature of 2758 °C and boil at 5400 °C. It is neither soluble in water and flammable in fire. It is also known as hafnia. This inorganic compound is one of the most common compounds of hafnium with stability. It reacts with concentrated sulfuric acid or other strong acids and gases, showing its inert characteristics. When it reacts with chlorine at high temperature with graphite or carbon tetrachloride, it produces hafnium tetrachloride. It is also an electrical insulator with 6 eV of band gap. [13]
Hafnium(IV) silicate (HfSiO)
Hafnium(IV) silicate has 270.57 g mol−1 of molar mass and its exact mass is 271.903133781 g mol-1. This compound can replace instead of silicon dioxide as a semiconductor and can be created through chemical vapor deposition. [14]
Hafnium(IV) chloride (HfCl4)
Hafnium(IV) chloride has monoclinic crystal structure with 320.20g/mol of molar mass and 432 °C of melting point. This organometallic compound works as a catalyst to lead to isomerism and alkylation reactions. It decomposes in water, but does not burn when it is exposed to fire. Its vapor pressure is 1 mmHg at 190°C. [15]
- Reactivity:
HfCl4 + 2 OC4H8 → HfCl4(OC4H8)2
2 HfCl4 + 2 K + 4 P(C2H5)3 → Hf2Cl6[P(C2H5)3]4 + 2 KCl
HfO2 + 4Cl + C → HfCl4 + CO2
RMg-X + HfCl4 → HfX4
Tantalum hafnium carbide (Ta4HfC5)
Tantalum hafnium carbide has the highest melting point, 4215°C. It is the hardest materials similar to tantalum carbide and hafnium carbide. Specially, the atomic hydrogen welding method by Irving Langmuir cannot fuse tantalum hafnium carbide by hammering or compressing. It is a refractory compound.[17]
Uses
- Nuclear power plants
- Nuclear fission reactors
- Binary compounds
- Scavenger metal to retrieve oxygen and nitrogen
- Electrode in plasma cutting
- Super alloys
- Refractory ovens/ lining furnaces
- Computer chip transistor
- Television/ Radio tubes
- Cathode in X-ray tubes
- Tantalum coating of rocket engines [7] [18]
Since Hafnium is a toxic and dangerous, it needs extra care while handling it. Breathing any compound of hafnium can lead into serious problem. Despite of its danger, people use hafnium in various ways. Among many usages, it is mainly used for nuclear power plants. The secondly common usage occurs in nuclear fission reactors by its characteristic of good reactivity with neutrons. Also, making rods in the fission reactor requires hafnium. Moreover, its refractory material characters makes it more useful in may fields.
Interestingly, in certain field, hafnium becomes a breakthrough for unsolved problems. 'Intel' and 'International Business Machines' (IBM) have decided to replace elements to make transistors with hafnium on January 26, 2010. To make smaller but more powerful microchips, the companies use different basic building blocks of microchip. [19]
‘We are getting down to a stage of technology where people have wondered if you could really ever go there, and we have definitely shown a roadmap down to these unbelievably tiny dimensions.’ said IBM’ Chief Technologist Mr.Bernie Meyerson.
Precaution and Cost
Similar to zirconium, hafnium needs extra careful awareness while using it. It can ignite suddenly in air and pyrophoric which means dangerous. The hafnium with purity does not act dangerous as much as compounds with hafnium, but still people needs to treat it carefully. However, people should not be exposured to this element no more than 0.5 mg/hr.
The costs of hafnium varies according to its percentage of purity and quantity. It varies from &100/lb to $500/lb. [3]
Energies
Ionization Energy
1st Ionization energy: 658.52 kJ mol-1
2nd Ionization energy: 1437.64 kJ mol-1
3rd Ionization energy: 2248.12 kJ mol-1
[9]
Thermal Properties
Heat of fusion: 25.5 kJ mol-1-1
Heat of vaporization: 570.7 kJ mol-1
Heat of atomization: 618.9 kJ mol-1
[9]
Isotopes
| Isotope | Atomic Mass | Half-life |
|---|---|---|
| Hf154 | 153.964 | 2 seconds |
| Hf155 | 154.963 | 0.89 seconds |
| Hf156 | 155.959 | 25 ms |
| Hf157 | 156.958 | 110 ms |
| Hf158 | 157.955 | 2.85 seconds |
| Hf159 | 158.954 | 5.6 seconds |
| Hf160 | 159.951 | 13.6 seconds |
| Hf161 | 160.9503 | 16.8 seconds |
| Hf162 | 161.9472 | 37.6 seconds |
| Hf163 | 162.947 | 40 seconds |
| Hf164 | 163.944 | 111 seconds |
| Hf165 | 164.945 | 76 seconds |
| Hf166 | 165.942 | 6.77 minutes |
| Hf167 | 166.943 | 2.05 minutes |
| Hf168 | 167.941 | 25.95 minutes |
| Hf169 | 168.9412 | 3.24 minutes |
| Hf170 | 169.94 | 16.01 hours |
| Hf171 | 170.94 | 12.1 hours |
| Hf172 | 171.9395 | 1.87 years |
| Hf173 | 172.941 | 23.6 hours |
| Hf174 | 173.94 | 2.0E15 years |
| Hf175 | 174.9415 | 70 days |
| Hf176 | 175.9414 | Stable |
| Hf177 | 176.9432 | Stable |
| Hf178 | 177.9437 | Stable |
| Hf179 | 178.9458 | Stable |
| Hf180 | 179.9465 | Stable |
| Hf181 | 180.9491 | 42.39 days |
| Hf182 | 181.9506 | 9E6 years |
| Hf183 | 182.9535 | 1.067 hours |
| Hf184 | 183.9554 | 4.12 hours |
| Hf185 | 3.5 minutes | |
| Hf186 | 2.6 minutes |
Video
Hafnium Valley: Intel's new Chip Hafnium - Periodic Table of Videos
References
- ↑ Hafnium Unknown Author, Lenntech Water treatment, Accessed Nov,16.2010.
- ↑ 2.0 2.1 Hafnium AZoM.com, AZO Materials, Dec 19, 2001.
- ↑ 3.0 3.1 Hafnium Unknown Author, Los Alamos National Laboratory's Chemistry Division, Last Updated Dec,15.2003.
- ↑ Hafnium Element Facts Unknown Author, by Chemicool, Accessed Nov,16.2010
- ↑ 5.0 5.1 Hafnium Unknown Author, 3rd1000.com, Accessed Dec.1.2010
- ↑ Hafnium Thomson Gale, Bookrags.com, Accessed on Nov.30.2010
- ↑ 7.0 7.1 7.2 Hafnium Unknown Author, Chemistry explained, Accessed Nov,30.2010
- ↑ Periodic Table of Elements - Hafnium Kenneth Barbalace, EnvironmentalChemistry.com, Accessed on-line: 11/26/2010
- ↑ 9.0 9.1 9.2 Hafinum Unknown Author, American Elements, Accessed Nov,30.2010
- ↑ Hafnium Wikipedia.org
- ↑ Hafnium diboride Wikipedia
- ↑ Hafnium carbide Wikipedia
- ↑ Hafnium oxide Wikipedia
- ↑ Hafnium silicate Wikipedia
- ↑ Hafnium chloride Wikipedia
- ↑ Hafnium Chloride Wikipedia, Worldlingo.com, Accessed Dec.2.2010
- ↑ Tantalum hafnium carbide Wikipedia
- ↑ Hafnium uses Sujata Iyer, Buzzle.com, July,19.2010
- ↑ [1] Intel Accessed on Jan.9, 2011
- ↑ Hafnium- The Future for Computer Chips Clippednews.wordpress.com
Additional Information
- Physical properties of hafnium-silicate transistor gate dielectric stacks after thermal processing Brendan Foran, Todd Rhoad, Michael Campin, Mark clark, Guoda Lian, Charlene Johnson, Gennadi Bersuker, Pat S. Lysaght, Cambridge Journals, Aug.1.2005
- Facts about Hafnium Unknown Author, Facts-about.org, Accessed Nov.30.2010
- Hafinum Unkwown Author, Mineral Information Institute, Accessed Nov,30.2010
- Use of Hafnium in Control Elements of Nuclear Reactors and Power Unit A. K. Shikov, O. V. Bocharov, V. M. Arzhakova, V. N. Bezumov, Yu. A. Perlovich and M. G. Isaenkova, Springlink, Accessed Nov.30.2010
| ||||||||||||||




