Selenium
| Selenium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General Info | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Symbol | Atomic symbol::Se | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Number | Atomic number::34 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Weight | Atomic weight::79.0 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical series | Nonmetals | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | gray-black, metallic luster 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 16, 4, p | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Ar] 4s2 3d10 4p4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 6 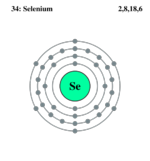
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | CAS number::7782-49-2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density | Density::4.79 g.cm-3 at 20°C g/ml | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | Melting point::217 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | Boiling point::688 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes of Selenium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| All properties are for STP unless otherwise stated. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Selenium is the 34th element in the Periodic Table of Elements. It's atomic symbol is Se, it's atomic number is 34, and it's atomic mass is 78.96 amu. Selenium was discovered in 1817 by Jons Berzelius. It's name originates from the Greek word 'selene' which means moon.
Properties
Selenium is a non metallic element with an atomic number of 34. It has chemical activity and physical properties similar to that of sulfur and tellurium and exists in several allotropic forms.[1] It has an atomic mass of 78.96, 34 electrons, and it's atomic symbol is Se.[2] An interesting property of selenium is that its ability to conduct electricity is affected by the amount of light shining on it. As the brightness of the light increases, the better that selenium conducts electricity.[3] It has a gray-black, metallic luster. It most closely resembles tellurium and sulfur when it comes to chemical activity and physical properties. Selenium dissolves in alkalis but is not effected by water.[4]
Occurrences
Selenium is quite uncommon and is harder to find then silver.[5] Though it is hard to find, it does occur naturally in the environment and is widespread but in small quantities.[6] Uncombined selenium can be found but it mostly occurs with sulfides of metals such as copper and zinc.[7] Today most of the selenium we obtain is as a byproduct of refining copper.[8] It is also gathered from the anode slimes that linger after the electrolytic refining of copper. The three most important forms in which selenium occurs are the noncrystalline, red crystalline, and gray metallic. The most stable of these is the metallic form.[9]
Uses
Selenium has many uses for the products we use everyday. Because selenium's ability to conduct electricity is affected by the brightness of the light shining upon it, selenium is used in many devices such as photo cells, electric eyes, and light meters for copiers and cameras. The red color in many enamels and glasses, an additive to stainless steel, and as a photographic toner. It also can create electricity from sunlight directly and for this reason it is used in solar cells.[10] The glass industry uses selenium to remove color from glass, the second largest use of selenium. An important function of selenium is its use in metal alloys like lead plates used in storage batteries and rectifiers to convert AC power to DC power. Anti-dandruff shampoos use some selenium compounds to aid in the prevention of dandruff.[11] In addition to its uses in electroning devices, selenium is a catalyst in many chemical reactions and used in organoselenium chemistry. For animals, selenium is an essential micronutrient.[12]
Essential to Humans
Selenium is an essential micronutrient to humans, though it is toxic in large doses.[13] A lack of selenium is known as selenium defficiency and can cause many dangerous health problems. The reason for this is that selenium is included in proteins to make selenoproteins, significant antioxidant enzymes. These assist in averting cellular damage from free radicals, by-products of oxygen metabolism that might contirbute to diseases like heart disease and cancer. Selenoproteins also aid in regulating thyroid function and have a part in the immune system.[14] The effects of selenium defficiency have been the subject of many studies. These studies suggest a link between selenium defficiency and diseases such as cancer and HIV/AIDS. Along with selenium defficiency, a study showed that the more selenium taken in, the higher risk of developing type II diabetes.[15]
References
- The Element Selenium Jefferson Lab, JLab.org, Thomas Jefferson National Accelerator Facility - Office of Science Education.
- Selenium (Se) Lenntech, Lenntech.com, Unknown author.
- Selenium Wikipedia, wikipedia.org, Multiple authors.
- Selenium Encyclopædia Britannica. 2008. Encyclopædia Britannica Online.
- Dietary Supplement Fact Sheet: Selenium National Institute of Health, Office of Dietary Supplements, NIH.gov
| ||||||||||||||

