Properties
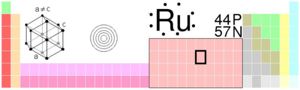
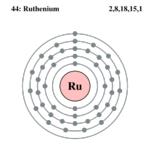
Ruthenium is a very rare transition metal, it belongs in group 8, period 5 on the table. Ruthenium, together with rhodium, palladium, osmium, iridium, and platinum form a group of elements commonly referred to as the platinum group metals also known as PGM. [1] Ruthenium is a hard silver-white metal, it does not tarnish/dull at room temperatures, The metal is unaffected by air, water and hot or cold acids, even a very strong acid called aqua regis. However when the common household supply, bleach (potassium chlorate) is added to the solution, it oxidises almost rapidly.This metal can be attacked by halogens, hydroxides, etc.[2]
It can be plated by electrodeposition (a process that uses electrical current to reduce dissolved metal cations so that they form a coherent metal coating on an electrode) or by thermal decomposition methods (a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes.). This metal is one of the most effective hardeners for platinum and palladium, and is combined with these metals to make electrical contacts for severe wear and tear resistance. Ruthenium is a suspected carcinogen (a substance capable of causing cancer in living tissue.) and its compounds strongly stain the skin. Touching raw Ruthenium would not be advised. Ruthenium tetroxide (RuO4), like osmium tetroxide, is highly toxic. In addition, it may explode. Ruthenium compounds show a marked resemblance to those of cadmium.
Uses
One of ruthenium’s primary uses is for wear-resistant electrical contacts and the production of thick-film resistors. Ruthenium is one of the most effective hardeners for platinum and palladium and is alloyed with these metals to make electronics and laboratory equipment. The corrosion resistance of titanium can be improved a hundredfold by the addition of 0.1% ruthenium. [3] Imagine what more of ruthenium could do, alongside that it is also a versatile catalyst, making it easier to mix with other elements. Hydrogen sulfide can be split catalytically by light using a watery suspension of cadmium sulfide ( a principle source of cadmium for all commercial applications) and particles loaded with ruthenium dioxide. Elemental or metallic forms of ruthenium include pellets, rod, wire and granules for evaporation source material purposes.
Recent experiments with ruthenium demonstrate functions that create new and unique properties and benefits. Ruthenium is also available in soluble forms including chlorides (a compound of chlorine with another element or group), nitrates (a salt or ester of nitric acid), and acetates (ester of acetic acid). These compounds can be manufactured as solutions it would not normally be necessary to make a sample of ruthenium in the laboratory as the metal is available, commercially. The industrial extraction of ruthenium is complex because this metal occurs in ores mixed with other metals such as rhodium, palladium, silver, platinum, and gold. Sometimes extraction of these metals is the main focus of a particular industrial operations while in other cases it is just a byproduct.
Video
Video description here....
References
- ↑ Ruthenium: the essentials ‘'Web Elements. Name Unknown Web.Date-of-access 20 oct 2014.
- ↑ Page-Title Lenntech. Author Unknown Web. Date-of-access 20 oct 2014.
- ↑ Page-Title ‘'Los Alamos National Laboratory . Author Unknown Web. Date-of-access 20 oct 2014.