Tungsten
| Tungsten | |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||
| General Info | |||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Symbol | Atomic symbol::W | ||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Number | Atomic number::74 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Weight | Atomic weight::183.8 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical series | Transition metals | ||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | grayish white, lustrous 
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 6(VIB), 6, d | ||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Xe] 4f14, 5d4, 6s2. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 12, 2 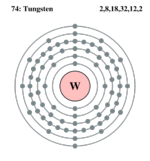
| ||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | CAS number::7440-33-7 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||||||||||||||||||||||||||
| Density | Density::19.3 g/ml | ||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | Melting point::3410°C | ||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | Boiling point::5530°C | ||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes of Tungsten | |||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||
| All properties are for STP unless otherwise stated. | |||||||||||||||||||||||||||||||||||||||||||||||||
Tungsten is a chemical element classified as a transition metal in the periodic table of elements. Its name is derived from Swedish words meaning "heavy stone". The chemical symbol of Tungsten is W, which stands for the original name for the element, Wolfram.
History
The existence of tungsten was first hypothesized by Peter Woulfe in 1779. He was studying a rock called Wolframite and concluded that it had to contain a new substance. In 1781 Carl Scheele found a new acid that could have been made from Tungstenite. Scheele and Torbren Bergman suggested it could be possible to isolate a new metal by reducing tungstic acid. [1]
In 1783 the Elhuyary brothers, Jose Elhuyary and Fausto Elhuyary, found an acid in Wolframite that was identical to tungstic acid. Later that same year, in Spain, the Elhuyary brothers succeeded in isolating tungsten by reducing the acid with charcoal and they were credited with the discovery of a new element, tungsten. During World War II, as Europe’s main source of tungsten at the time, Portugal was pressured by both sides to distribute tungsten for the making of weaponry.[1]
Properties
Pure tungsten varies in color from steel- gray to tin white. Only pure tungsten can be cut, formed or shaped due to its strength. Impure forms of tungsten can be very brittle and hard to work with.[2] When tungsten metal is exposed to air, it forms a protective oxide [1] a compound in which oxygen is bonded to one or more electropositive atoms.[3] The most common oxidation number is +6, but it can also show all oxidation numbers from -1 to +6. Like most elements, Tungsten mostly combines with oxygen forming a yellow colored compound called tungstic oxide, WO3[1]
Occurrence
Tungsten is found deep in the Earth's crust and is estimated to be 1.5 grams per one ton of rock. Tungsten does not occur as a free metal and is about as abundant as tin or molybdenum and half as plentiful as uranium. While China is reported to have over 75% of the Earth's tungsten resources, California, Colorado, South Korea, Bolivia, and Russia also have important deposits of tungsten. During World War II two of Portugal's tungsten mines in Panasqueira and Borralha allowed miners to extract 6000 tons of ore and rock [4] per year. [5]
Uses
Tungsten is used in many objects in today’s world. Tungsten is used to make electric lamps and high temperature applications for spacecraft.[2] Cement carbide is the most important use for tungsten; its main component is Tungsten carbide (WC). It has the strength of cast iron and makes sharp cutting tools for steel.[6] Tungsten is also found in turbine blades in aircraft,[7] steel,[8] and diamond tools. [9] Because Tungsten has a high tolerance for heat and is incredibly strong, this metal is mixed with many other metals for engineering. Tungsten was one of the first elements to be used systematically to improve steel alloy properties and has been added since the middle of the twentieth century to quicken the speed of tools, cutting efficiency and the hardness of the metal. The main use for tungsten is to make tools for cutting, working, shaping, and forming metals. [8]
At the beginning of the twentieth century, tungsten was put in the lamp making process instead of osmium. Because of osmium's high vapor pressure, the metal could not withstand the high temperature in a lamp. Tungsten has a considerably lower vapor pressure and could take the high temperature of the lamp. When the wiring within a light bulb was made with osmium, the wire would get so hot that it would stretch until it would touch the glass of the bulb resulting in a bad light. Tungsten can be strung into a thin light bulb wire, but once within the light bulb the wire will not quickly stretch much further in the heat. Tungsten is now used in nearly all lamps and light bulbs, especially household lamps, automotive lamps, and reflector lamps. [10]
Tungsten is often used in aircraft engines and marine vehicles because of its strength at high temperature and good welding properties. The metal within any engine gets hot when the vehicle is on, working or moving. When airplanes are in the air, the engine tends to reach temperatures higher than normal. When most metals gradually get warmer and warmer, they tend to stretch and slow down its function. Tungsten is put in aircraft engines because the engine does not produce enough heat for the metal to creep. Tungsten is also mixed in with tantalum to increase the high- temperature strength and creep resistance. [7]
During World War II, there was a high demand of Tungsten made products and tools, of the United States and European Army's causing a shortage of Tungsten. Steel makers found an alternative metal called molybdenum as a replacement. [8] Molybdenum has many of the same characteristics as that of Tungsten. Molybdenum has a high temperature tolerance, much like Tungsten, and has a silvery white color to it. [11]
According to the North American Tungsten Corporation Ltd. Tungsten is mostly used in metals in Western Europe, Japan and the United States, but in China Tungsten is mostly used in making steel and alloys than anything else. Ammonium Paratungstate (APT) is the main metal used in its raw and intermediate state in the trade market. Tungsten is used as a power source as well. To make Tungsten metal power, yellow or blue oxide is reduced to Tungsten metal power by way of hydrogen by either in a pusher furnace, then the power passes through to a furnace in a boat, or in a rotary furnace at 700- 100 °C.
After Tungsten metal powder is produced, most of it will be converted to Tungsten carbide by the reaction of pure carbon powder put in a pusher furnace at 900- 2,200°C, this process is called carburization. Tungsten carbide is the most important Tungsten compound because of its hardness and it contribution in making hard metals. [12]
Other products that Tungsten is used for are drills for drilling oil and gas. Also drill bits for mining, construction, radiation shielding and wear-resistant tools of metalworking. [13]
Biological, Health and Environment Roles
As Tungsten is harvested from the Earth's crust and processed, studies have shown that the by- produces generated have low potential in causing harm to animals and humans. During Tungsten production the pollutants are gathered by filters, recycled and then sold as individual products. This keeps the losses of Tungsten at a minimum and this presence will improve as economic forces will require the individual by- produces ammonia, and sodium sulphate, of Tungsten. The products that are not or cannot be filtered often help the environment by cleaning the exhaust fumes, saving fossil fuels, and is a substitute for toxic lead.
While Tungsten processing helps the ecosystem, Tungsten is an essential nutrient of some organisms. Enzymes named oxidoreductases use Tungsten by using it in a Tungsten- pterin complex.[1] But there is a link between oxidoreductases in leukemia and Tungsten that causes leukemia. [14]
The amount of Tungsten found in tree rings from Fallon, Nevada increased between 1990 and 2002, while the amount in tree rings from nearby cities stayed the same, according to a research team led by Paul R. Sheppard of The University of Arizona's Laboratory. This is the first study that has examined changes in levels of heavy metals in Fallon over a long period time. "Trees take up metals from the environment and those metals show up in the tree rings. By analyzing chemicals in tree rings, we can look back in time years," Paul R. Sheppard Tungsten levels in Fallon trees began increasing in 1994, while levels in nearer by towns remained the same. Since 1997, seventeen cases of childhood leukemia have been diagnosed in children who lived in the Fallon before their diagnosis. Fallon's many cases of leukemia has been labeled as a leukemia cluster by the Nevada State Health Division.
In a 2003 U.S. Health and Human Services report investigating possible causes for the leukemia cases in Fallon. Tungsten was mentioned as "a contaminant of concern because it was elevated in urine samples". Prior to research by Sheppard and his colleagues found elevated levels of Tungsten and cobalt in airborne and surface dust in Fallon as well. When Tungsten particles, less than 10 micrometers in diameter, are inhaled they have the potential to cause health problem. Researchers think that oxidoreductases are living in blood and survive on Tungsten, which once in the blood, cause leukemia. [15]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 .Tungsten by Wikipedia, Accessed January 17, 2011.
- ↑ 2.0 2.1 Tungsten Unknown Author, Los Alamos National Laboratory's Chemistry Division, Last Revised 12/15/2003.
- ↑ Oxide Dictionary.com, Dictionary.com, 2010
- ↑ Ore Definition Dictionary.com, LLC. Copyright 2010.
- ↑ Tungsten Mining China Tungsten Online, Unknown Publisher, Accessed Nov Mon 15, 2010.
- ↑ Tungsten - W Lenntech Water treatment & purification Holding B.V., Unknown Publisher, 1998-2009
- ↑ 7.0 7.1 Tungsten Alloys International Tungsten Industry Association, Unknown Publisher, 2005
- ↑ 8.0 8.1 8.2 Tungsten in Steel International Tungsten Industry Association, Unknown Publisher, 2005.
- ↑ Diamond Tools International Tungsten Industry Association, Unknown Publisher, 2005
- ↑ .Lamps Unknown Author, International Tungsten Industry Association, 2005
- ↑ .What is Molybdenum Conjecture Corporation, Unknown Publisher, 2003 - 2010
- ↑ .Tungsten Uses North American Tungsten Corporation Ltd., Unknown Publisher, Accessed Mon Nov 15, 2010
- ↑ .Tungsten- Minerals and Uses Mineral Prospector Unknown Publisher, Accessed Mon Nov 15, 2010
- ↑ .Leukemia Blood, American Society of Hematology, Unknown Publisher, 2002,
- ↑ .Elevated Tungsten In Trees Science Daily, Science Daily LLC, 1995-2010
| ||||||||||||||








