Protactinium
| Protactinium | |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||
| General Info | |||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Symbol | Atomic symbol::Pa | ||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Number | Atomic number::91 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Weight | Atomic weight::231.03588 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical series | actinide | ||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | bright,silvery metallic luster 
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | group n/a,period 7,f-block | ||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Rn] 5f2,6d1, 7s2 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2,8,18,32,20,9,2 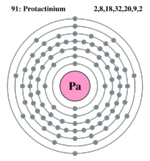
| ||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | CAS number::7440-13-3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||||||||||||||||||||||||||
| Density | Density::15.37 g/ml | ||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | Melting point::1841 K | ||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | Boiling point::4300 K | ||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes of Protactinium | |||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||
| All properties are for STP unless otherwise stated. | |||||||||||||||||||||||||||||||||||||||||||||||||
Protactinium is a chemical element identified by the chemical symbol Pa. It is one of the rarest and most expensive of all naturally occurring elements. It cannot be used commercially because their high toxicity and radioactivity.
Properties
Protactinium appears bright color, and it has a silvery metallic luster. Also, it can sometimes maintain its own color in the air condition. This element has superconductivity (the ability of certain metals to allow electricity to pass through them without any resistance at very low temperatures) when temperatures fall below to 1.4K (minus 457.2 F). [1]
It can easily react with water vapor and oxygen. Also, inorganic acids can be diverse compounds. When the protactinium develops compounds, it can appear most balance in the oxidation (the deposit that forms on the surface of a metal as it oxidizes) state +5 because it can be easily hydrolyze. Therefore, hydroxide ions combine together, and it changes into a kind of soluble or insoluble hydroxyl-oxide solids. However, it can also appear in +4, +3 and +2 oxidation states too. Protactinium has an alpha emitter (a radioactive isotope that emits alpha particles), and it occurs with 5.0MeV. In addition, this element is radioactive, which makes it dangerous. When handling this element, extreme caution is necessary. [2]
Occurrences
Protactinium is one of the rarest naturally occurring elements. Commonly, the average concentrations of protactinium in the Earth's crust is about parts per trillion. Its rarity makes it one of the most expensive natural elements. Discoveries of it are about a few parts per million in some uraninite ore deposits. [2] Also, one part of 231Pa occurs 10 million per pitchblende uranium ores. Ores have an extent of 3 ppm from the Democratic Republic of the Congo.[3]
Uses
Protactinium-231 is commonly used in radio-date marine sediments combined with thorium-230. These elements originated from the uranium. In addition, they have different isotopes but their properties and rates show extreme similarity. They can be used dependably in radio-date marine sediments down through 175.000 years because of their half-life. However, protactinium is a marginal element in our life, and it cannot be used in commercially because it is dangerous. They have a high toxicity and radioactivity. Therefore, it is only seen in a research laboratory. [4]
History
Protactinium was first discoverd by Kasimir Fajans and O.H. Göhring in 1913. When they were studying uranium's decay chain, they found particular isotope things. It was protactinium-234m, which spends time with 1.7 minutes in its half-life. In that time, this element had a different name. The name was brevium, which meant "brief". Then other people continued with their studies. After time passed, protactinium's existence was proved by two groups of scientists in 1918. They were Otto Hahn and Lise Meitner of Germany, and Frederick Soddy and John Cranston of Great Britain. They found another isotope and it was protactinium-231. The first time protactinium was isolated was by Aristid V. Grosse in 1934. [5]
Video
This is explanation of protactinium.
References
- ↑ Helmenstine, Anne.Protactinium facts about education. Web. Accessed October 10, 2014.
- ↑ 2.0 2.1 Facts About Protactinium livescience. Web. Last-update September 17, 2013. Author Unknown.
- ↑ Protactinium Protactinium. Web. Accessed October 26, 2014. Author Unknown.
- ↑ Element of the week: protactinium. GrrlScientist. Web. Last-update June 14, 2013. Author unknown.
- ↑ Gagnon, Steve. The Element Protactinium. Jefferson Lab. Web. Accessed October 10, 2014.
| ||||||||||||||
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||


