Electrochemistry
Electrochemistry is the branch of chemistry that deals with the chemical action of electricity and the production of electricity by chemical reactions. Of particular importance is the interface between an electron conductor (typically a metal electrode) and an ionic conductor (the electrolyte).
Electrolytic Cell
- Main Article: Electrochemical Cell
Electrolytic cells are used in electroplating, electrorefining, and even when recharging rechargeable batteries[1]. Electrolytic cells use electrical energy and store it as chemical energy, causing chemical reactions that wouldn't normally occur at that temperature. The electrolytic cells are like Voltaic cells because they use the two kinds of chemical reactions, reduction reactions and oxidation reactions. In an Electrolytic cell these two reactions are driven by a source of electrical power.
Voltaic Cell
Voltaic cells or Galvanic Cells are electrochemical cells that change chemical energy into electrical energy through the redox reactions.
In one example of a wet cell, we can see zinc and copper [2] which form the two electrodes and also dissolve in water. The oxidation reactions occur on the anode (the zinc electrode which is the negative side of the battery), making positive ions which lose electrons which can then flow out of the anode. The copper cathode is where reduction occurs (the copper electrode is positive). It is the copper electrode where positively charged ions arrive to be "reduced" by reacting with (negatively-charged) electrons arriving at the cathode[1]. Therefore, the electrons flow from the anode to the cathode. Electrons are moving all the time to keep the reaction balanced.[3]
When reduction or oxidation occurs, the metal ion in each part of the reaction is a half-cell, and these half-cells are connected by a salt bridge. The salt bridge allows charge to move from one half-cell to the other but not completely mixed together. We usually use some metal like platinum as the conducting wire to allow the cell to discharge electricity.
The activity series of metals determines which half-cell oxidizes and usually the more active metal is the one oxidized during the reaction.
Dry Cell
People use voltaic cells as energy sources, and the commonly used dry cell is a voltaic cell in a paste electrolyte. The flashlight battery is one common dry cell. In the dry cell, the moist paste contains MnO2, ZnCl2, NH4Cl, and H2O. The normal flashlight battery uses zinc in the container and this zinc is also the anode of the battery; the graphite rod which resides in the moist paste and acts as the cathode.
This is the half-reaction for the cells:
Oxidation: Zn--->Zn2++2e- (anode reaction)
Reduction: 2MnO2+2NH4++2e---->Mn2O2+2NH3+H2O (cathode reaction)
The graphite rod only serves as conductor and does not experience reduction in the ordinary dry cells.
Lead Storage Batteries
We use lead storage batteries in cars. There are six voltaic cells in one 12V car battery. Each voltaic cell produces 2V of electric potential. In the lead storage battery, the plates are grids of solid lead with square holes filled with a lead oxide paste made by mixing the lead oxide with dilute sulfuric acid.[4] Negative plates are made like the positive plates but with the addition of small amounts of expanders (such as the wood flour originally used) to the paste to make certain that the lead doesn't solidify.[5] The pastes help ensure that the grids are filled with spongy material that allow the acid electrolyte to penetrate easily in the anode and cathode plates.
The half reactions are:
- Oxidation:Pb+SO4--->PbSO4+2e-
- Reduction:PbO2+4H++SO4+2e---->PbSO4+2H2O [6]
When the battery is fully charged, the negative plates are nearly pure lead, while the positive plates are nearly pure lead oxide. With continuing discharge both the lead and lead oxide slowly turn into lead sulfate in the solution. When the battery is recharged, the lead returns from solution to the plates.
Electrical Potential
People use electrical potential to measure the ability of a cell can produce an electric current. We cannot measure two separate half-cells, but we can measure the difference between them, which called the cell potential, by using this formula:
Cell Potential= reduction potential in reduction - reduction potential in oxidation or E0cell= E0red - E0oxid
Standard Cell Potential
In standard cell potential, the E0red represent the standard reduction potential for reduction, and E0oxid represent the standard reduction potential for oxidation.
The standard reduction potential of the hydrogen electrode at 25 degrees is 0.00V. Usually shows as E0H+=0.00V
Standard Reduction Potential
Usually when we need to determine the standard reduction potential of some half-cells, can use the standard reduction potential of reduction subtract the standard reduction potential of oxidation, which shows in this formula:
E0cell= E0red- E0oxid
Electrolytic vs. Voltaic Cells
Electrolytic cells can use electrical energy to change the chemical properties. The electrons inside the electrolytic cells need an outside power to push them completing the flowing in the reaction, but the voltaic cells do not need a outside power to do this. [7]
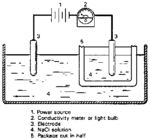
Electrolysis of Water
When we add a low concentration acid into pure water, the electrolysis might happen and the products of the electrolysis of water are the hydrogen gas (H2) and oxygen gas (O2).
Reduction: 2H2O+2e---->H2+2OH- (cathode)
Oxidation: 2H2O--->O2+4H++4e- (anode)
The region that around the cathode and anode turns to basic and acidic because the OH- and H+ we got from the half reactions. [8]
Uses of Electrolysis
People usually use electrolysis in metal processing.[9]
Electroplating
There are a lot of plated objects in our daily life, especially jewelry. Jewelry is usually plated with gold or platinum to make them have more value and be better looking. Basically, electroplating just places a thin metal layer on the outside of the products and this layer might help the object from corrosion.[9]
Electrowinning
Electrowinning is a way to purify metals using electrolytic cells.[9]
Electorefining
By using electorefining we could produce pure metals and determine the impure ones.[9]
References
- ↑ Electrolysis - Electrolytic Cells Aus-e-tute. Accessed 8 July 2010.
- ↑ Electrochemical Cells Hyperphysics. Accessed 8 July 2010
- ↑ Wilbraham, Staley. Prentice Hall Chemistry. 2008. Pearson Education Inc., publishing as Pearson Prentice Hall, Boston, Massachusetts. p665.
- ↑ Lead Battery Wikipedia. Accessed 8 July 2010.
- ↑ The Constructing of the Lead Acid Batteryby Eugene P. Finger and Dr. David P. Boden, Curtis Instruments 2007. Accessed 8 July 2010
- ↑ Wilbraham, p669.
- ↑ Wilbraham, p679.
- ↑ Wilbraham, p680.
- ↑ 9.0 9.1 9.2 9.3 Wilbraham, p682.
Additional Information=
- Electrolytic Cells by Bodner Research Web
- Voltaic Cells by R.H. Logan, Instructor of Chemistry, DCCCD
- Demonstration of a Voltaic Cell by Blackgold
- Dry cell by Wikipedia
- The Dry-Cell Battery by Library.kcc.hawaii.edu
| ||||||||||||||