Sulfur hexafluoride
| Sulfur hexafluoride | |
|---|---|
| |
| General | |
| Systematic name | Hexafluoro-λ6-sulfane |
| Other names | Elagas, Esaflon, Sulfuric fluoride |
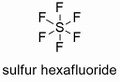
| Molecular formula | F₆S |
| Molar mass | 146.06 g/mol |
| Appearance | colorless |
| CAS number | 2551-62-4 |
| Properties | |
| Density and phase | 0.01 g/cm³, ? |
| Solubility in water | 0.003% (25 °C) |
| Melting point | −64 °C |
| Boiling point | −50.8 °C |
| Acidity (pKa) | 0.1 ppm |
| Basicity (pKb) | ? |
| Chiral rotation [α]D | ?° |
| Viscosity | ? cP at ?°C |
| Structure | |
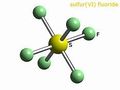
| Molecular shape | octahedral |
| Coordination geometry |
Orthogonal hexagonal |
| Crystal structure | Orthorhombic, oP28 |
| Dipole moment | D |
| Hazards | |
| MSDS | Material safety data sheet |
| Main hazards | ? |
| NFPA 704 | |
| Flash point | ?°C |
| RTECS number | WS4900000 |
| Related compounds | |
| Other anions | ? |
| Other cations | ? |
| Related [[]] | Disulfur decafluoride, Sulfur tetrafluoride |
| Related compounds | Selenium hexafluoride, Sulfuryl fluoride, Tellurium hexafluoride |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Disclaimer and references | |
Sulfur Hexafluoride is a very interesting gas that has many properties and uses that make certain jobs easier and in some cases less dangerous. Sulfur Hexaflouride is a synthetic gas that was discovered in 1901. The discovery of Sulfur Hexafluoride has changed the medical and electrical industries in some cases. There have also been other thing that without Sulfur Hexafluoride, would not have been possible.
Properties
Chemical
Sulfur Hexafluoride or SF6 is an inert, colorless, odorless, and inorganic gas that has one sulfur atom surrounded by six fluorine atoms. Sulfur hexafluoride is one of the most stable gases, it can remain stable up to 500 degrees Celsius.
Physical
Sulfur Hexafluoride is an invisible gas that is also one of the heaviest gases. It is about six times heavier that air. Due to sulfur hexafluoride being an inert gas which is safe to inhale and that it is six times heavier that air, if you breath it in your voice will become extremely low and unnatural. Sulfur Hexafluoride is also known the opposite of helium.
Synthesis / Occurrences
Sulfur Hexaflouride is synthetically make by combining sulfur one atom with six fluorine atoms. Sulfur hexaflouride is an inorganic gas that does not occur naturally.
Uses
Sulfur Hexafluoride is used as a test gas in respiratory phycology, as well as a contrast agent in and ultrasound. During an ultrasound the gas is distributed through the lungs in order to get a clearer image. This gas is commonly used as a filler between panes of glass in an insulated window. It is also used for casting magnesium. inn the electrical industry, Sulfur Hexafluoride is use when the electrician does not want an arc to travel form onw pace to another due to its ability to block the flow of electricity through the air.
Other
If a large container is filled with Sulfur hexafluoride, then a light aluminum foil boat can float on to of the gas, giving the appearance that the boat is floating on nothing.
Video
Video description here....
References
| ||||||||||||||
- ↑ SF6 Gas Properties Electrical4u.com . Web. March 3, 2018. unknown author
- ↑ Sulfur hexafluoride Drugs.com. Web. March 6, 2018. Author Unknown
- ↑ Sulfur hexafluoride Pub Chem. Web. September 16, 2004. Unknown Author



