Periodic table trends
Periodic table trends are specific patterns that are present in the periodic table of elements that illustrate different aspects of a certain element, including its size and its electronic properties. These trends tell us different aspects of the elements and how they compare to each other. They include Atomic Radius, Ionic Radius, Electron Affinity, Electronegativity, and Ionization Energy. [1]
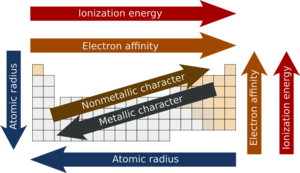
Atomic Radius
Atomic radius refers to the size of a certain atom. This term Atomic Radius is also related to Ionic Radius, Metallic Radius, or Covalent Radius. What factor decides how big or small the atom will or can be? There is one answer for this question. Electrons. The size depends on how far the most outer electron is to the Nucleus. The only way anyone could measure the Atomic Radius is by taking two Atoms and making them touch. Then find the difference between the two nuclei and split that distance in half.[2]
How is the Atomic radius used on the periodic table? The size of the atoms decrease in size as we move from left to right in a diagonal manner. This is because all the electrons become more and more and more packed close together as we go left to right on the table. On the other hand, when we go down the column in a vertical manner the Atomic Radius increases. From all this information, we should now know that the largest Atomic Radius is at the very bottom left of the table and the smallest Atomic Radius is at the very top right of the periodic table.[2]
Ionic Radius
Do you know what ionic radius means? If not, then you are reading the correct article. Ionic radius is the radius of an Atom's ion structure. Its very difficult to measure. It is measured in either Picometers (pm) or Angstroms (A). 1 A is equal to 100 pm. When the ions are negative the Ionic Radius is much larger but when the ions are positive they aren’t as large. The Ionic radius falls as we move across a row or period. When there are positive ions, they have the same structure with makes them isoelectronic. The distance can also be measured by x-ray crystallography (the study of crystals and their structure by means of X-ray diffraction). This gives the correct length of the sides of a cell of the crystal.[2]
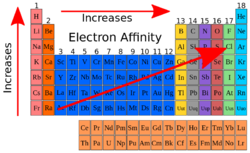
Electron affinity
The electron affinity is described as the change in the energy the atom has when an electron is added to it because of a chemical change which then forms a negative ion. It becomes a negative ion because an electron is added to the atom. But, if the atom would lose an electron then it would be a positive atom. When moving down a group the Electron affinity becomes less negative, but across a period it can either decrease or increase depending on what the electron configuration of the element is. If an element doesn’t have 8 electrons but seven, it should gain one electron and then it becomes a element with a negative charge. But if the element is in the second row and only has two electrons for instance beryllium, it would be a positive 2 charge because it only has 2 electrons so it must lose them. [3]
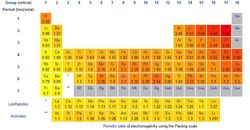
Electronegativity
The term electronegativity is defined as a measure of the impulse that an atom has that attracts a bonding pair of electrons. A fun fact is that the most Electronegative Element is Fluorine, but the least Electronegative element are Cesium and Francium. The most common scale to measure Electronegativity is the Pauling scale. Let’s say that two Atoms want to bond together but they both have the same Electronegativity, they have the same impulse to attract each other so that means that they would be called a pure Covalent Bond. On the other hand, if that one Atom is a little more Electronegative but one is less, the one that was more Electronegative will to have a little negative charge but the one that was less Electronegative is going to have a little positive charge. This would be described as a Polar Bond, because it is a Covalent Bond where both Atoms charges are changed. But if one has a much more high Electronegativity than the other, this will produce a ionic bond. Electronegativity can’t not make a fully pure or non-pure Covalent Bond. A small Electronegativity will produce a Covalent Bond, and a large Electronegative difference will produce an Ionic Bond. As we move across the Periodic Table the electronegativity increases and as we move up it also increases. In summary the top right is the most electronegative and the bottom left is the least electronegative.[4]
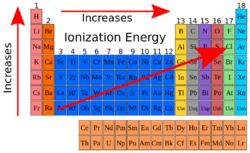
Ionization Energy
What is Ionization Energy? You must be thinking that it involves some sort of energy because energy is in the name. If you did think that, you are right! The correct definition of Ionization energy is “The energy that is required to take away or to remove an electron out of a gaseous atom or an ion". The initial ionization energy or Ei of the atom or ion is the energy that it takes to take out one mole( a chemical mass unit, defined to be 6.022 x 1023 molecules, atoms, or some other unit. The mass of a mole is the gram formula mass of a substance.) of electrons from gaseous atoms. the lower the ionization energy level is then the easier it is to remove an electron, thus the higher the energy level is then the harder and more difficult it is to remove an electron. This energy can help people or chemists to predict the strength of the chemical bonds. Also, this type of energy is basically a clue of reactivity.
Ionization energy can be known as Ionization potential as well. How do we measure this energy? First off, Ionization Energy is shown or measured in unites such as Kilojoule per Mole or Electron Volts. This trend will increase in the Periodic Table moving left to right across a row and it will decrease moving top to bottom in a group. The reason behind this is that the Atomic Radius decreases across a row which means that there would be a larger attraction between the Positive and Negative Atoms. The Noble gases have a full valence shell so it retains from this trend, and it is at its weakest at the Alkali Earth Metals. The farther we travel down a group the more protons there will be found. The first Ionization Energy is used to remove electrons from the first group of elements called Alkali Earth Metals. But the second and so forth uses the second Ionization energy which requires a higher level of Ionization Energy.[5]
Video
This Video Explains different aspects of The Periodic Table of Elements.
References
- ↑ Pauling, Linus. Periodic table of the elements Encyclopedia Britannica.. Web. Last modified January 3, 2018.
- ↑ 2.0 2.1 2.2 Clark, Jim. Atomic and Ionic radius Chem Guide. Web. Last-Modified August, 2012.
- ↑ Clark, Jim. Electron Affinity Chem Guide. Web. Last-Modified August, 2012.
- ↑ Clark, Jim. Electronnegativity Chem Guide. Web. Last-Modified March, 2013.
- ↑ Anne Marie, Helmenstine. Ionization energy definition and trend thoughtco. Web. Last-Modifided February 10, 2017 .
| ||||||||||||||