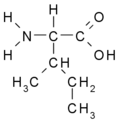
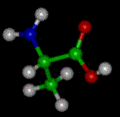
Isoleucine
| Isoleucine | |
|---|---|
| |
| General | |
| Systematic name | 2-amino-3-methylpentanoic acid |
| Other names | H-DL-ILE-OH |
| Molecular formula | C6H13NO2 |
| SMILES |
Canonical SMILES: |
| Molar mass | Molar mass::131.17 g/mol |
| Appearance | White crystalline powder |
| CAS number | CAS number::73-32-5 |
| Properties | |
| Density | Density::1.035g/ml |
| Solubility in water | 4.117 g/100 ml (25°C) |
| Melting point | Melting point::210°C |
| Boiling point | Boiling point::225.8°C |
| Molecular shape | Missing Goalpost |
| Hazards | |
| MSDS | Material safety data sheet |
| NFPA 704 | |
| Flash point | 90.3°C |
Isoleucine (typically abbreviated as 'I' or 'Ile') is an essential amino acid, meaning the body cannot make the molecule, but rather it must be obtained from food. It comprises a significant part of the protein in muscle tissue where it helps muscles to be used at their full capability and is important for wound recovery. It is therefore not surprising that athletes need more isoleucine than other people.
Properties
Isoleucine is a natural amino acid that is an essential nutrition for the human body. The chemical property of isoleucine is aliphatic and the physical property of isoleucine is nonpolar. Standard 70 kg people need to consume about 650 - 700 mg of isoleucine per day. The molecular shape is missing goalpost. Isoleucine is C-beta branched like Valine and Threonine. Isoleucine has two non-hydrogen substituents, while most amino acids have one non-hydrogen substituent that is attached to their C-beta carbon. This means that isoleucine has more limits to form than the main-chain can adopt. 'Ile' and 'I' refer isoleucine. Isoleucine is stable and a strong oxidizing agent.
Isoleucine has seven different forms; isoleucine, D-isoleucine, L-isoleucine, DL-isoleucine, allo-D-isoleucine, allo-L-isoleucine and allo-DL-isoleucine. Synonyms for D-isoleucine, L-isoleucine, DL-isoleucine and allo-DL-isoleucine are (R)-Isoleucine, L(+)-Isoleucine, (R*,R*)-isoleucine and alloisoleucine respectively. People can have a lack and high intake of isoleucine. People have headaches or are dizzy or depressed when they do not have enough isoleucine in their bodies. Even when people intake isoleucine too much, they do not get any harmful effects but it may cause problems with their kidney or liver disease. One needs a doctor's advice to take isoleucine.
Occurrences
Isoleucine can be easily obtained in a wide variety of foods. Egg, meat, chicken, nuts, soy beans and fish has lots of isoleucine. Isoleucine is also formed as cereals and seeds to eat comfortably.
Isoleucine cannot be made in the body by itself, therefore people have to get isoleucine by consuming food. Foods that include isoleucine have high-protein. For instance, nuts, seeds, meats, eggs, fishes, lentils, peas, and soys have good isoleucine. We do not have to find isoleucine in the foods listed above because people are producing isoleucine in supplements. Especially for the people who work out or exercise a lot, there are isoleucine pills that they can purchase in stores. Since isoleucine is an important amino acid and since the body cannot manufacture it, we need to obtain isoleucine by eating. Scars or wounds will not be easily healed when people do not have enough isoleucine. Muscles also need isoleucine to work at 100% of their capability. In conclusion, one will be very weak without isoleucine.
Uses
Isoleucine, valine, and leucine holds responsible for one-third of all the protein in muscle tissue. It claims that isoleucine is an essential amino acid for the human body. Isoleucine is very important to build muscle and increase energy in muscle cells. Isoleucine provides energy and power to move the body or muscles. Athletes use isoleucine to hone their ability to perform. Isoleucine is also used by athletes to heal and repair their muscles, skin and bones. It can also be used to regulates the blood sugar levels and the production of hemoglobin.
References
- Isoleucine Basic Information about Isoleucine (National Institute of Standards and Technology)
- Property of Isoleucine Properties of isoleucine (Amcaid)
- Isoleucine, Valine and Threonine Isoleucine and close acids (Robert B. Russell, Matthew J. Betts & Michael R. Barnes)
- Solublity of Isoleucine Solubility of isoleucine in water(AminoScience)
- Use of Isoleucine Use of Isoleucine (ReaonsToBelieve)
- Common Use Use of Isoleucine in real life (nutritiondynamics)
- Supplements of Isoleucine Use of Isoleucine in our bodies (OnliveVitaminGuide)
- Symtompt of Wrong Uses of Isoleucine Lack and over-take of Isoleucine (HealthVitaminGuide.com)
| ||||||||||||||

