Ionic crystal
Ionic crystals are crystals formed by ions that hold together by electrostatic forces (ionic bonds). Ionic crystals are hard and have relatively high melting points. Table salt (NaCl) is an example of this type of crystal. They also include most of the alkali halides and many metal oxides (MgO, NiO).
Structures
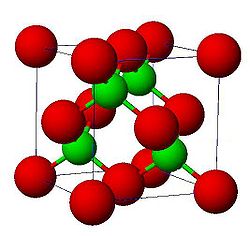
Ionic crystals have at least two atoms in their base which are ionized. Charge neutrality requires that the total charge in the base must be zero; so the base atoms always need some ions with an opposing charge. For the binding of the structure, it doesn't need direction because they use strong ions which are electrostatic. These ions are doing multiple things at once. The crystal acts like it wants to have a minimum amount of volume while minimizing electrostatic energy. In an Ionic crystal, there are no free electrons so Ionic Crystals are insulators. Ionic crystals come in different lattice types, some are straightforward and simple while others can be more complex and complicated. The latter is true in particular for oxides which are often counted among ionic crystals. A simple lattice is a center faced cube, with two atoms at the base. One of the atoms is at (0,0,0) and the other is at (½,0,0).
Types
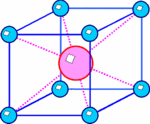
In Ionic Crystals there are multiple different types of structures. First there is the NaCl structure. The NaCl Structure has many salts and oxides in it. Then there is the CsCl structure. The lattice of this structure type is a cubic primitive with two atoms in the base at (0,0,0) and (½, ½, ½). It is easy to mistake it for a bcc lattice. Another type is ZnS structure. This type of lattice is a face centered cube with two atoms in the base at (0,0,0) and (¼, ¼, ¼). It is not only an important lattice for other ionic crystals like ZnS, from which it got its name, but also the typical lattice of covalently bonded group IV semiconductors (C [diamond form], Si, Ge) or III-V compounds semiconductors. The ZnS lattice can also be mistaken with the ZrO2 lattice.
History
According to underlying atomic forces, crystalline solids are put into five classes: molecular, ionic, covalent, metallic, and hydrogen bonded. These classes tend to blend into each other, but still represent ideal types. Ionic crystals are a class of crystals in which the lattice site are charged ions. These are the easiest type of chemical bonding to visualize since it is almost totally electrostatic in the natural state.
Video
References
| ||||||||||||||