Ethylene glycol
| Ethylene glycol | |
|---|---|
  | |
| General | |
| Systematic name | Ethane-1,2-diol |
| Other names | Monoethylene glycol, 1,2-ethanediol |
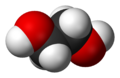
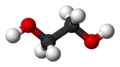
| Molecular formula | C2H6O2 |
| SMILES | occo |
| Molar mass | Molar mass::62.0678 g/mol |
| Appearance | Clear oily odorless viscous liquid |
| CAS number | CAS number::107-21-1 |
| Properties | |
| Density and phase | [[Density::1.1132 g/cm3]], liquid |
| Solubility in water | Miscible with water |
| Melting point | Melting point::-12.9°C |
| Boiling point | Boiling point::197.3°C |
| Viscosity | 17.33 Pa.s at 25°C |
| Structure | |
| Dipole moment | 0 D |
| Hazards | |
| MSDS | Material Safety Data Sheet |
| Main hazards | Highly toxic if inhaled or absorbed through skin |
| NFPA 704 | |
| Flash point | 111°C |
| R/S statement | R: R22, R36 S: S26, S36, S37, S39, S45, S53 |
| RTECS number | KW2975000 |
| Related compounds | |
| Related compounds | Propylene glycol Diethylene glycol Triethylene glycol |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Disclaimer and references | |
Ethylene glycol is an organic compound containing a dihydric alcohol that is commonly known as an ingredient in automotive antifreeze, but also has many other uses. It is a relatively inexpensive chemical that is used in many industries around the world. It was not found until 1859 by a French chemist Charles Wurtz. Later in 1937, it was formally introduced to the automotive industry as a heat transfer liquid.[1]
Properties
Ethylene glycol is identified as an compound containing carbon. It is colorless, clear and odorless liquid. It is composed of a dihydric alcohol that has an aliphatic carbon chain and two –OH diols. These two –OH diols give ethylene glycol its water solubility and hygroscopicity. It has a very rare characteristic, which is a sweet taste.[2] Its sweet taste attracts children and pets to consume the liquid in excess, unlike any other toxic liquids which could normally be bitter. When consumed in excess there is a possibility of serious or even fatal toxicity.[3]
Production
Ethylene glycol is not naturally found in nature by itself. Rather ethylene glycol is produced by a chemical reaction between organic compounds such as ethylene. Many times this reaction takes place between glycol plant and an organic compound. Ethylene glycol is commonly produced from ethylene. [4] In 1860 ethylene glycol was first produced by the hydration of ethylene oxide. [5] Even today, the hydration of ethylene glycol serves as the most common process of producing ethylene glycol. [6] There are also many other ways to produce ethylene glycol. The ethylene carbonate process uses a reaction of ethylene oxide to react with carbon dioxide. Then it is hydrolyzed to produce ethylene glycol. Later this process became obsolete in 1970, due to the finding of the ethylene oxide-glycol plants.[7] In the halcon acetoxylatin process, ethylene is placed in an acetic acid solution producing ethylene glycol diacatate. This reaction liquid is processed which forms glycol acetates which are later hydrolyzed to form ethylene glycol and acetic acid. In the teijin oxychlorination process ethylene reacts with thallium salt with the hydration of water and chloride or bromide ions. The final process is the union carbide syngas process which uses synthetic gas to produce ethylene glycol.[8]
Uses
Ethylene glycol has a very diverse field of uses. One of the main uses of this chemical is as an coolant and heat transfer liquid. This may include automotive radiators, hydraulic brake fluid, liquid cooled computers, air conditioning systems, and many other heating and cooling applications. The number one use of this product is in automotive radiator coolant. When ethylene glycol is added to water the boiling point increases and the freezing point decreases. This then provides a worthy heat transfer liquid during hot and cold days. [9] Also it is used in airports as a aircraft and runway deicer. In addition, ethylene glycol is used in the production of a polymer called poly(ethylene terephthalate) or PET. This polymer is due to the reaction between the ethylene glycol and the terephthalate acid. Thus this polymer opens up a ample spectrum of uses in both the food container and textile industries.[10][11]
Antifreeze
Antifreeze is a cryoprotectant (a substance that is used to protect organic tissue from freezing over). Ethylene glycol is the major ingredient in antifreeze. When it is added to water, because of its solubility it creates a heat transfer liquid. This then increases the boiling point of the water and also decreases the freezing point. Antifreeze is a vital chemical in the function of the average automobile. Not only does it help reduce the heat generated by the engine, it also helps reduce the corrosion in the radiator and in the engine block. [12] These corrosion inhibitors are 0.05-0.5 percent silicate, from 0.07-0.35 percent nitrate, from 0.2-2.0 percent phosphate, from 0.5-4.0 percent benzoate, from 0.1-1.0 percent molybdate, from 0.02-0.3 percent vanadate, from 0.05-0.3 percent triazole, and from 100-5000 ppm organosilane stabilizer for the silicate. The main importance is to use the recommended amount of antifreeze to reduce the risk of engine failure. [13]
In addition there is only one drawback to ethylene glycol constructed antifreeze; its toxicity. It has been used as a fatal poison because its sweet taste, which allows it to be mixed into a drink undetected. When antifreeze is drained onto the ground without care, it may be consumed by animals. On the average every year, 90,000 animals and 4,000 children ingest the toxic liquid. When the symptoms are not recognized the lethal ingestion of the liquid can lead to death. The lethal limit of ethylene glycol antifreeze is 1.4-1.6 mL/kg. [14][15]
References
- Properties of Ethylene glycol By www.sbioinformatics.com
- Chemical facts Mallinckrodt Chemicals MSD
- Production of ethylene glycol By www.sbioinformatics.com
- Ethylene glycol toxicity and uses By www.epa.gov
- Ethylene glycol uses By www.science.jrank.org
- Ethylene antifreeze Brendan I. Koerner
- Corrosion inhibitor By www.freepatentsonline.com
- Ethylene glycol poisoning By www.antizol.com
- Ethylene glycol history By www.spiritus-temporis.co
| ||||||||||||||


