Ammonium chloride
| Ammonium chloride | |
|---|---|
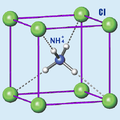
| |
| General | |
| Systematic name | Ammonium Chloride |
| Other names | Sal ammoniac, Salmiac, Nushadir salt,
Sal armagnac, Salt armoniack |
| Molecular formula | H4ClN |
| SMILES | [NH4+].[Cl-][1] |
| Molar mass | Molar mass:: 53.49 g/mol−1 |
| Appearance | White solid, hygroscopic |
| CAS number | CAS number::12125-02-9 |
| Properties | |
| Density and phase | [[Density::1.5274 g/cm3]] |
| Solubility in water |
244 g/L (−15°C)
294 g/L (0°C) |
| Solubility |
Soluble in liquid ammonia, |
| Melting point | Melting point::338°C |
| Boiling point | Boiling point::520°C |
| Acidity (pKa) | 9.24 |
| Hazards | |
| MSDS | Material safety data sheet |
| NFPA 704 | |
| Flash point | Non-flammable |
| R/S statement | R: R22, R33 S: (S2), S22 |
| RTECS number | BP4550000 |
| Related compounds | |
| Other anions |
Ammonium fluoride |
| Other cations |
Sodium chloride |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Disclaimer and references | |
Ammonium chloride is a white, crystalline, inorganic compound, known commonly for its use in batteries, applications in metal working, and medicinal values. Existing as either a natural or synthetically created compound, ammonium chloride (also known as sal ammoniac) is a common mineral. Although it is typically used in a solid state, it can occur in a gaseous state as well.
Properties
Physical
Ammonium Chloride is an odorless, fine particulate, white crystalline solid salt. This inorganic chemical compound with a molar mass of 53.49 g/mol is soluble in liquids such as water, methanol, and ethanol and insoluble in acetone, ether, ethyl acetate.[1] Sal ammoniac, as it is commonly called, has a molecular formula of H4ClN and is a result of the combination of ammonia (NH3) and hydrochloric acid (HCl). Ammonium chloride can be dangerous if it contacts your body. It will often affect the skin, eyes, and respiratory system, causing irritation, coughing, and difficulty breathing. Depending on what area is affected, safety procedures vary. When ammonium chloride contacts the skin, it is advised to simply wash the skin with soap and water. In the eyes, the CDC recommends rinsing the eyes with water, and if inhaled, the person should move outdoors for fresh air. [2]
Chemical
Ammonium Chloride is a non-flammable compound, but when exposed to high temperatures (such as those in a fire), harmful gasses may be released. These dangerous gasses can quickly corrode most metals.[3][1]
Synthesis / Occurrences
Ammonium chloride occurs naturally and can also be synthetically created. Sal ammonia commonly forms in nature near volcanoes where the hydrogen chloride gas from the volcano mixes with the ammonia in the soil. This process results in crystallized ammonium chloride. In fact, Mount Vesuvius remains one of the prolific natural sources of H4ClN.[4] The development of ammonium chloride may also occur as a result of chemicals that react when coal burns.[5]
The process of synthetically producing ammonium chloride begins with combining water and ammonia gas. This reaction results in ammonium hydroxide, which can then be combined with hydrochloric acid to produce ammonium chloride.[4]
Uses
Ammonium chloride is highly versatile with uses ranging from an ingredient in cooking, an electrolyte in dry-cell batteries, a component of certain hair products, and several applications in metal working and electrical industries. Although it can be harmful in larger quantities, the trace amounts in medicines and cooking ingredients are harmless.[4][5] This compound is even found in certain squid species to assist in buoyancy.[6]
Cooking: Occasionally, sal ammoniac is used as a flavor enhancer in cooking[7]. Mostly used in bread baking, ammonium chloride acts as a conditioner in the dough and a nutrient for yeast.[5][7]
Batteries: One of the most notable uses of Ammonium chloride is its use in the manufacturing of dry cell batteries. As an ionic compound, it can be used as an electrolyte (liquid or solution that conducts electricity)[5]. With more modern solutions and better electrolytes, ammonium chloride use in batteries is decreasing. Regardless, ammonium chloride is important in the battery industry. [8]
Soaps: Ammonium chloride can be found in soaps, shampoos, detergents, conditioners, and hair coloring products to increase the viscosity of the item.[7]
Metal Working: Ammonium chloride serves as an extremely beneficial chemical compound in the field of metal working. It is commonly used in solder or solder flux, and metal etching. The more common metalworking application, soldering, takes advantage of ammonium chloride's separating into ammonia and hydrochloric acid when heated. The hydrochloric acid then eliminates most metal oxides present on the surface and allows solder to successfully create the bond between materials. [9]
Medicinal Purposes
Ammonium chloride is also used for certain medical and pharmaceutical purposes. In different forms, sal ammoniac can be used as a dietary supplement, or an expectorant (stimulates the production of phlegm) in cough medicine[4]. As a dietary supplement, sal ammoniac balances pH levels within the body. The ammonium replaces the sodium within the kidneys in order to maintain the acid-base balance.[10] The ammonium chloride, taken in pill form, may improve the body's ability to retain nitrogen and regulate levels of blood, urea, nitrogen, uric acid, and creatinine.
Ammonium chloride's most common application in the medical field is its use in cough syrups or expectorants. As an expectorant, ammonium chloride stimulates the production of phlegm in the bronchial or laryngeal mucus membranes. The phlegm is then coughed up. In cough syrup, this salt acts in a very similar manner, allowing for a safe treatment for coughs. When too much of this cough syrup is ingested, negative side effects including nausea, vomiting, headaches, and drowsiness may occur.[4]
Video
This video displays the reaction of ammonia (NH3) and hydrochloric acid (HCl) to produce ammonium chloride.
References
- ↑ 1.0 1.1 1.2 Ammonium Chloride Chemspider. Web. Accessed 13 January 2015. Author Unknown
- ↑ Ammonium chloride fume Centers for Disease Control and Prevention. Web. Updated November 18, 2010. Author Unknown.
- ↑ Material Safety Data Sheet Ammonium Chloride Iowa State University. Web. Updated 8/02/2000. Author Unknown.
- ↑ 4.0 4.1 4.2 4.3 4.4 What Is Ammonium Chloride? WiseGEEK. Web. Accessed 13 January 2015. Author Unknown
- ↑ 5.0 5.1 5.2 5.3 Ammonium Chloride The Chemical Company. Web. Accessed 13 January 2015. Author unknown
- ↑ Giant Squid WWF. Web. Accessed 22 February 2015. Author Unknown.
- ↑ 7.0 7.1 7.2 Ammonium Chloride Cosmetic Info. Web. Accessed 27 January 2015. Author unknown.
- ↑ Dry Cell Battery Boundless. Web. Accessed 26 January 2015. Author Unknown
- ↑ Ornitz, Barry, L. Soldering Flux Usenet Archives. Web. Published 30, October 1999.
- ↑ Ammonium Chloride Drugs.com. Web. Accessed 27, January 2015. Author Unknown
| ||||||||||||||





